Source | A drug Buff
In December 2021, Pfizer COVID-19 took the medicine orally. Paxlovid Obtain FDA emergency use authorization.
In February 2022,Paxlovid It was approved by emergency conditions in National Medical Products Administration, China, and then it was written into the latest diagnosis and treatment plan.
Paxlovid It has been approved for listing in more than 50 countries around the world.
but Paxlovid Its use in the real world is full of controversy.
"I thank the pharmaceutical companies for providing patients with effective COVID-19 therapy. But I am also frustrated that these companies seem to be in complete control of the situation, and I believe that Americans can get more benefits from pharmaceutical giants as long as our regulators dare to ask. Paxlovid was approved based on a study that seems to exaggerate the benefits that most Americans can get from this drug, rather than providing us with more information about it. " This is a primary care doctor at Weill Cornell Medical College in new york when talking about Covid-19’s therapeutic drugs. Paul Fenyves Some feelings.
Among the antiviral drugs in COVID-19, Pfizer’s Paxlovid Has become an obvious winner for two reasons:
Firstly, compared with monoclonal antibodies and Remdesivir, Paxlovid, as a pill, is easier to administer.
Secondly, Paxlovid seems to be very effective. A clinical trial shows that,Hospitalization or death of high-risk patients who received treatment decreased by 89%..
It seems to be very effective because there is a problem: FDA’s emergency use authorization (EUA) for Paxlovid is only based on the data of a single trial, and the trial only includes people who have never been vaccinated with COVID-19 vaccine before. At present, 76% of American adults have been vaccinated with COVID-19 vaccine, and it is estimated that 58% of Americans have been infected with Covid-19, so the experiment supporting Paxlovid EUA is not directly applicable to most Americans.
This means that doctors who treat patients with COVID-19 do not know to what extent (if any) Paxlovid can benefit patients who have been vaccinated. Doctors only have hope and speculation, and continue to prescribe Paxlovid to high-risk patients in COVID-19, but they are not sure whether they are really helping them.
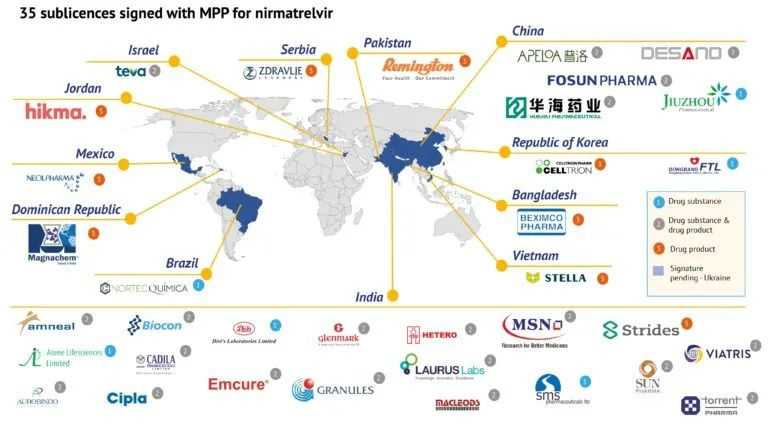
Paxlovid Generic has been authorized in 95 countries.
History seems familiar.
Paxlovid’s current situation is reminiscent of previous antiviral drugs.oseltamivirIt was also controversial during the outbreak of swine flu.
Influenza kills an average of 40,000 Americans and hundreds of thousands of people around the world every year, so everyone is trying to develop antiviral drugs that can reduce its severity. Roche first introduced it in 1999.oseltamivir (oseltamivir), it is such a drug. Clinical studies reported by Roche show that existing evidence shows that Tamiflu reduces the risk of pneumonia, which is a common and important complication of influenza. For many years, doctors’ impression of using Tamiflu is that it reduces the risk of serious diseases.
Tamiflu (oseltamivir phosphate capsule) is indicated for the treatment of influenza A and B in adults and children over one year old. Patients should use it within 48 hours of the first symptom.
Tamiflu was included in the list of essential drugs by WHO. During the influenza outbreak in 2009, governments all over the world spent a lot of money to reserve a lot of Tamiflu to protect their citizens, because its manufacturer said, "Trust us, it works!" .
The Cochrane Collaboration made a meta-analysis of the clinical trial data of oseltamivir (Tamiflu) and zanamivir (Legonqing of GSK) and all published literature research results, and formed a comprehensive report, which was published in the British Medical Journal (BMJ) on April 10th, 2014.
The report pointed out that, oseltamivir can reduce the proportion of symptomatic influenza in prevention research.It can shorten the time needed for symptom relief in treatment research; However, there is no obvious effect in improving the hospitalization rate, and there is no strong evidence to support the reduction of influenza complications such as pneumonia, which has no obvious effect in reducing the spread of the virus. At the same time, it also questioned the practice of many countries in the world to reserve Tamiflu to prevent influenza pandemic.
In response to these reports, Roche issued a statement saying that it disagreed with the overall conclusion of Cochrane Collaboration Group. This report did not include all the data about Tamiflu, "only included 20 research data from 77 clinical trials they had mastered, and excluded the actual data obtained from observational trials".
However, in 2017, WHO adjusted Tamiflu from "core drug" in the list of essential drugs to "supplementary drug", and the applicable population was hospitalized critically ill patients who were diagnosed or suspected of influenza.
Although many doctors are skeptical about Tamiflu, they are like Paul Fenyves Similarly, they can’t ignore a drug that may be helpful to patients, especially the drug approved by the US Centers for Disease Control and Prevention. Therefore, doctors continue to prescribe Tamiflu to patients with high-risk influenza, hoping that the advantages outweigh the disadvantages.
Paxlovid’s data
Paxlovid’s situation is not exactly the same as Tamiflu’s, but there must be some "historical reappearance": doctors have to make clinical decisions on antiviral drugs for respiratory viruses without sufficient data.
There are two noteworthy facts about the people who participated in the Paxlovid trial. They were not vaccinated with Covid-19 vaccine and had never been infected with Covid-19 before.
First of all, even if the Paxlovid trial began in July 2021, only a few Americans were in the category of unvaccinated and uninfected with Covid-19, so the test results were never directly applicable to most Americans.
Secondly, Paxlovid is obviously more effective for people who have not been vaccinated with COVID-19 vaccine or have never been infected before, so the experiments supporting its use exaggerate the benefits that most people see from the drug.
Maybe you will say that Pfizer has started to test Paxlovid in high-risk population who have been vaccinated with COVID-19 vaccine, yes, but this trial is a mixture of vaccinated and unvaccinated patients, which may confuse the problem. More importantly, the test results will not be released until November 2022.
On April 29th, Pfizer released a phase II/III clinical (EPIC-PEP) study of Paxlovid for post-exposure prevention. The results show that,Paxlovid failed to reach the main end point, that is, effectively preventing the infection of family members who had contact with confirmed patients in COVID-19.. Therefore, Paxlovid has no preventive effect.
Who cares?
Pfizer’s first experiment exaggerated the efficacy of Paxlovid, which is not surprising, because as a pharmaceutical company, it has endless motivation to maximize its influence and promote sales.
But to everyone’s surprise, the US federal government will buy Paxlovid worth $5 billion, without Pfizer’s quick action to answer this basic question: "How does Paxlovid perform in high-risk groups who have been vaccinated with COVID-19 vaccine or previously infected?"
Paxlovid costs $530 (about 3,500 yuan) for a five-day course of treatment, but if there is no proper data, it is impossible to judge whether it is a good deal or a bad one. For example, if it is necessary to prescribe 10,000 courses of Paxlovid for people over 65 who have been vaccinated with COVID-19 vaccine to prevent one hospitalization, then a government may not place an order immediately; On the contrary, if 10 people can avoid a hospitalization by taking Paxlovid, they will be in a hurry to buy more.
However, if we don’t know the performance of Paxlovid in people who have been vaccinated with COVID-19 vaccine, it is impossible to make such calculations.
In addition, given the history of Tamiflu, the requirements for data transparency and independent review should be cautious.
Some people may say, even if we finally learn that Paxlovid’s benefits from people who have been vaccinated or infected before are greatly weakened, who cares?
But this problem is a symbol of a bigger problem: we lack the much-needed independent review of drug clinical trials.
Americans have seen scandals caused by poor supervision. When Covid-19 appeared, the pharmaceutical industry performed exceptionally well, providing safe and effective vaccines in record time. But before that, many Americans had "learned" not to trust Big Pharma, so many people (tragically) rejected this life-saving intervention.
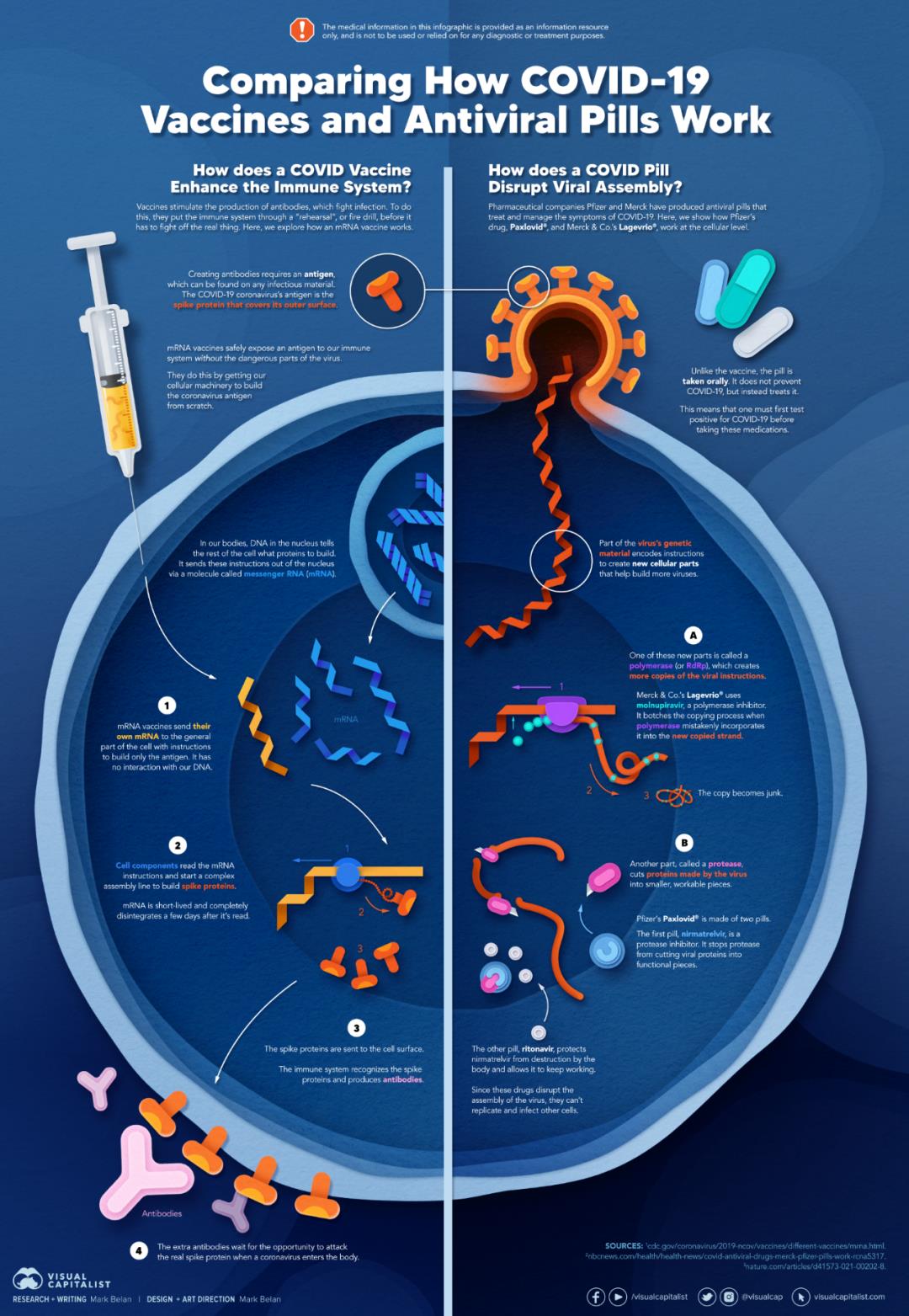
Comparison of Action Mechanism between COVID-19 Vaccine and Oral Medicine
The discussion around "vaccine indecision" mainly focuses on false information, but what is missing is to admit that the pharmaceutical industry has wasted public trust for decades.
Instead of continuing to feel depressed about people who have not been vaccinated, we should reflect on ourselves and then start rebuilding the public’s trust step by step, which will be a long-term work.
关于作者